Notifications
ALL BUSINESS
COMIDA
DIRECTORIES
ENTERTAINMENT
FINER THINGS
HEALTH
MARKETPLACE
MEMBER's ONLY
MONEY MATTER$
MOTIVATIONAL
NEWS & WEATHER
TECHNOLOGIA
TV NETWORKS
VIDEOS
VOTE USA 2026/2028
INVESTOR RELATIONS
DEV FOR 2025 / 2026
ALL BUSINESS
COMIDA
DIRECTORIES
ENTERTAINMENT
FINER THINGS
HEALTH
MARKETPLACE
MEMBER's ONLY
MONEY MATTER$
MOTIVATIONAL
NEWS & WEATHER
TECHNOLOGIA
TV NETWORKS
VIDEOS
VOTE USA 2026/2028
INVESTOR RELATIONS
DEV FOR 2025 / 2026
About Me
 Mindy Hausler
Mindy Hausler Alfa Chemistry is a leading supplier and manufacturer of raw materials, fine chemicals, reagents and life science products. We are focused on providing high-quality customer service, whilst delivering the highest quality products all around the world to meet customers' diversified needs.
 Mindy Hausler -
7 hours ago -
Health -
Azide PEG
Bioorthogonal Chemistry
Drug Delivery
Nanoparticle Functionalization
Synthesis Methods
-
24 views -
0 Comments -
0 Likes -
0 Reviews
Mindy Hausler -
7 hours ago -
Health -
Azide PEG
Bioorthogonal Chemistry
Drug Delivery
Nanoparticle Functionalization
Synthesis Methods
-
24 views -
0 Comments -
0 Likes -
0 Reviews
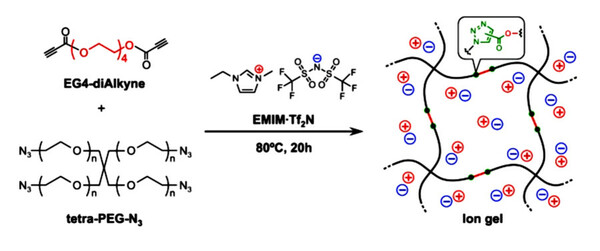
Azide PEG, or azido-functionalized polyethylene glycol, is a class of hydrophilic polymers with azide (-N3) groups covalently bound to PEG chain termini or side chains. These azide groups acilitate precise bio-orthogonal reactions through copper-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC) to build stable triazole bonds. Structurally, azide PEG can be designed as monofunctional forms such as azido-PEG-NH2 or bifunctional forms like azido-PEG-azido and advanced multi-arm versions for intricate conjugation methods.
The PEG backbones feature outstanding water solubility alongside chemical durability and biocompatible properties. Azide PEG products typically have molecular weights that fall between several hundred and tens of thousands of Daltons while maintaining narrow polydispersity indices under 1.05, which results in consistent performance and predictable pharmacokinetics. The fundamental structure-function relationship establishes azide PEG as an essential material for use in bioconjugation and pharmaceutical applications.
Alfa Chemistry provides a broad selection of azide PEGs that feature precisely defined structures and superior purity levels for use in both academic settings and industrial research environments.
The process to create azide PEG uses a two-phase methodology.
A. Polymer Backbone Construction
The synthesis of PEG chains occurs through ring-opening polymerization of ethylene oxide, which allows precise control over molecular weight and terminal functionalities. The choice between monomethoxy PEGs and dihydroxy PEGs as starting materials depends on where the azide group needs to be positioned.
B. Azide Functionalization
Azide groups are usually introduced through nucleophilic substitution reactions. Tosyl chloride or mesyl chloride reacts with terminal hydroxyl groups on PEG chains to generate a tosylated intermediate that then undergoes reaction with sodium azide (NaN3) under mild conditions. Researchers perform the reaction in polar aprotic solvents while monitoring conditions to prevent excessive substitution and chain breakdown.
Following synthesis, scientists conduct purification through dialysis or column chromatography alongside recrystallization to remove azide salts and other impurities that remain.
Azide PEG facilitates bioorthogonal conjugation with precision and efficiency because its terminal azide groups participate readily in 1,3-dipolar cycloaddition reactions. CuAAC stands out as the most recognized reaction for producing 1,4-disubstituted 1,2,3-triazoles through copper(I) catalysis with excellent regioselectivity. Alternatively, SPAAC reactions do not use toxic copper catalysts, making them ideal for live cell applications and in vivo conjugation.
This selective chemistry allows azide PEG to serve as a molecular linker in attaching therapeutic agents, imaging probes, targeting ligands, or surface-functionalizing agents to nanoparticles and biomacromolecules. The combination of robust reaction conditions with rapid processing times and high yields leads to minimized off-target effects and improved functionalization process efficiency.
Drug Delivery Systems
Azide PEG serves as a modular platform for designing targeted drug carriers. By linking cytotoxic drugs, ligands, or antibodies via triazole linkages, the resulting conjugates exhibit improved solubility, enhanced circulation time, and reduced immunogenicity. PEGylated drug carriers such as liposomes, micelles, and dendrimers functionalized with azide groups offer enhanced pharmacokinetics and controlled drug release profiles.
Gene Therapy Vectors
Azide PEG can be conjugated to cationic polymers or lipids to create non-viral gene carriers with tunable surface chemistry. By modifying vectors with azide PEG, researchers can enhance DNA/RNA complex stability, minimize aggregation, and introduce targeting moieties using click chemistry.
Imaging and Diagnostics
Azide PEG is extensively used for the synthesis of bioimaging probes. Azide-functionalized PEGs are conjugated with fluorophores or radiolabels for applications in fluorescence imaging, PET, and SPECT. Their hydrophilic nature ensures improved bioavailability and a low non-specific background signal.
Nanoparticle Surface Modification
Functionalization of nanoparticles with azide PEG improves colloidal stability, reduces protein corona formation, and allows for site-specific attachment of targeting molecules. This is critical for creating stealth nanoparticles that evade immune detection.
Tissue Engineering and Regenerative Medicine
In scaffold design, azide PEG provides a chemically versatile platform for attaching bioactive peptides, ECM proteins, or cross-linkers to hydrogels. These engineered materials can mimic biological cues and support cell adhesion, proliferation, and differentiation.
Biosensors and Molecular Probes
Azide PEG is used in the design of molecular probes and biosensors. Click chemistry enables the precise attachment of recognition elements such as antibodies, aptamers, or enzyme substrates to sensor surfaces, allowing for high-sensitivity detection of biological analytes.
The molecular design modifications of azide PEG influence its pharmacological activity and physical chemical properties. Key variables include
The design requirements must correspond directly to the intended purpose through examples such as nanocarriers' drug-loading efficiency, imaging agents' stealth properties, or hydrogels' crosslinking density.
